/in
In 4 weeks I'll finish all the organic chemistry I have left. It's proteins and sugars. I knows basics about everything else.
Organic CHEMISTRY-may need replacement, come /in to replace
-
vezokpiraka

-
vezokpiraka Jack of All Trades
 vezokpiraka
vezokpiraka
- Jack of All Trades
- Jack of All Trades
- Posts: 6034
- Joined: June 17, 2010
-
vezokpiraka

-
vezokpiraka Jack of All Trades
 vezokpiraka
vezokpiraka
- Jack of All Trades
- Jack of All Trades
- Posts: 6034
- Joined: June 17, 2010
Isn't what we define as organic kind of wonky?In post 52, Antihero wrote:
there are a number of compounds that are referred to as "bleach". most of them contain some form of hypochlorite, but none of them are organic.In post 44, Jingle wrote:Also, can anyone tell me if bleach is an organic compound?
I mean CCl4 is considered organic, but HCl is anorganic.Windows hasn't detected any keyboard. Press Enter.-
vezokpiraka

-
vezokpiraka Jack of All Trades
 vezokpiraka
vezokpiraka
- Jack of All Trades
- Jack of All Trades
- Posts: 6034
- Joined: June 17, 2010
-
vezokpiraka

-
vezokpiraka Jack of All Trades
 vezokpiraka
vezokpiraka
- Jack of All Trades
- Jack of All Trades
- Posts: 6034
- Joined: June 17, 2010
I know this stuff. I was just pointing out that the distinction between organic and inorganic is weird and not very useful.In post 61, Antihero wrote:
right, but the carbon and hydrogen aren't bonded. the proton goes onto one of the oxygens.In post 59, vezokpiraka wrote:Yet CO2 is inorganic.
Even HCO3- is inorganic and it contains both carbon and hydrogen.
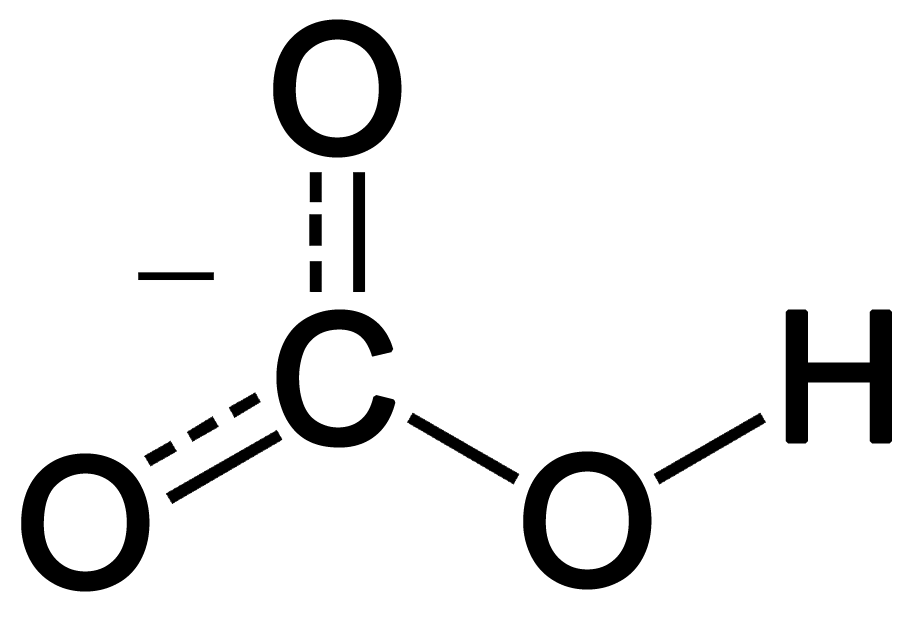
for something to be organic, it needs "C--H" bonds. i put that in quotes because things get kind of weird sometimes. for example, CCl4 is organic because the C--Cl bonds can be derived from C--H bonds and kind of behave like them (kind of).
...yeah, it's weird.Windows hasn't detected any keyboard. Press Enter.
Copyright © MafiaScum. All rights reserved.